Last month we talked a little about the mysteries (and effects) of airflow. This time, how about some conversation regarding gas flow? No, not the kind you get from a 99 cent fast food menu, or ported heads, or even from a gas pump into your Harley’s fuel tank. What we’re talking about this time is a phenomenon a lot less known and just beginning to be understood as a major impact on fossil fuel efficiency.
No matter what the hype regarding alternatives for our own personal locomotion, we are stuck with fossil fuel for a long time to come. Make no mistake about that. Electric, steam, hydrogen, solar, natural gas and who knows what other “motive” power has been tried and rejected—never perfected—and long neglected for many a good reason. Even hybrid cars (which actually strike me as massively compromised long-term alternatives, since they pack two motors yet never let you use both) have to tank up on dead dinos, now and then. That being the case, along with the certain knowledge that the status quo can’t last and that future costs (of all definitions) are uncertain to say the least—and you have every motive in the world to improve upon what you get as much as you can.
You’ve heard that gasoline isn’t what it used to be, haven’t you? Most of us (and we know who we are) can actually remember a time when you could pump seriously high-octane (like Sunoco 104) and not call it race gas. Forget that it was cheap—the stuff was good! Nowadays, “premium” (ha!) gets you a paltry 92 octane rating (91 in California), and something close to 10 percent alcohol mixed in with who knows what kinds of nasty chemicals and other additives that might prove worse for us than the lead they took out decades back. It just ain’t a pretty picture, yet we presume it’s all we have and “make do.”
History does repeat, and those who refuse to learn from it do suffer. Case in point, fuel has had its ups and downs over the last century, to put in mildly. For instance, after World War II the British were still stuck with so-called “pool petrol” as a vestige of the war effort. The stuff was created to stretch supplies for the duration of the conflict, and rationed like crazy to boot, yet no one in their right mind would choose to run such a low grade (70 octane on a good day) today—even if they sold it at 1945 prices! Obviously, engines of the era were tuned (detuned, more like) to operate on it, with power and longevity suffering accordingly. Amongst the many subtle knee-jerk outcomes of the second global clash, ironically, was the rapid return of high-compression engines and higher octane ratings, clear up through the 1960s! The horsepower race, which basically began in earnest with the advent of the small-block Chevy V8 in the mid-’50s, was in a way the direct outcome of better fuels. After a decade-long lull, starting with the fuel crisis of 1972 and coinciding with the first EPA mandates for cleaner air (and to “get the lead out”) today’s ongoing power race is more a case of efficient combustion chamber shapes and computers on board than anything else. The technology largely works around current fuel “qualities” (or lack thereof). Fine for cars, even lots of water-cooled motorcycles maybe, but not so much for our beloved, essentially anachronistic hogs.
Perhaps the most important lesson in the history of gasoline is the first one.
Though they work together, octane and energy in gasoline aren’t the same! The wizard of GM, Charles F. Kettering, proved this almost 100 years ago. Octane is there to extract more work from the energy available, via a bigger “push” when the mixture burns in a high-compression engine. The concept to take away from this tidbit is that it took Kettering (and Midgley) to prove that the limitations were in the fuel, not in the engines. Everyone else thought otherwise at the time. Back then, it was not a question of working around fuel quality with applied mechanical technology—they just didn’t have that technology. The solution was chemical. Surprise, surprise! Well, it just could be that we’re back to that situation again—history repeating, so to speak.
So much for octane, what about energy?
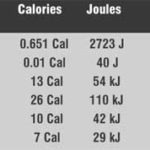
The energy in a gallon of gasoline is measured in so many ways it can become confusing—Joules, calories, and more—even on occasion being compared to foods. I’ve read that a gallon of fuel has the energy content of 110 McDonald’s hamburgers—although it’s not clear whether that’s a basic burger or a Big Mac. (Either way, definitely a description of two major types of gas!) Still, it’s not condusive to any form of energy measurement. Hell, we even get bogged down in defining the quantities of the stuff in which we look for that mysterious mass of energy—is that Imperial gallons, American gallons, liters or what? Still, however inaccurate the “yardstick” we offer some measurements to compare in grams.
The different materials on our little chart are distinguished as much by the rate at which they release their energy, as by how much they contain—however it’s measured! That’s important!
These characteristics are why you tend not to use gasoline to blow things up, you don’t use TNT to run your motorcycle (or car), and butter will melt to nothing way before you’d ever get it to explode. Fact is there’s a distinction between an explosion, in which a nearly instantaneous, often devastating “shock wave” is generated, and a slower, more controlled “conflagration,” with no shock wave. There is no explosion in normal internal combustion—no shock wave in the burning of gasoline in an engine. So by this definition, it’s the “conflagration” that matters!
Burn baby, burn!
It stands to reason that to get the most energy and efficient “work” from any fuel, complete, controlled combustion is the goal. All that should be left behind is heat. Rate of conflagration is one thing—think firewood vs. dynamite, for instance—but lots of factors can affect those rates. Water, to name one, will mess up both wood and dynamite, as wet stuff simply doesn’t burn as well (or fast) as dry stuff, right? Well, one of the biggest factors in terms of effective combustion of gasoline is static electricity! It’s actually becoming more and more apparent that this might be the largest remaining inhibitor to getting the most bang for your buck out of a gallon of so-called “pump” gas.
We already know that high mechanical compression ratios, and the discovery and implementation of anti-knock compounds (octane boosters) in fuels were effectively “work-arounds” to address the “conflagration” problems of pure petroleum gasoline. What if the next breakthrough was simply a way to reduce or eliminate the static “cling” in modern gas? Static electricity does strange enough things to other everyday objects. We’ve all rubbed a balloon on the carpet, then stuck it on the ceiling or a wall and watched it mysteriously “cling” there. We also know ladies’ slinky silk skirts look better when they swing rather than cling. Ever walked across a room to turn on a wall switch and caught a spark from the damn thing? (Yup, thought so!) Well, variations of all of these common occurrences resulting from static “charges” are present in some form or another in the gasoline we use every day. Gas can pass current through itself, cling to the walls of your fuel tank and/or worse, stick (like that balloon to the wall) and refuse to flow through your injectors! That same laminar boundary layer we discussed last month in relation to airflow (and water down a drain) is present in gasoline. More to the point, static also creates surface tension within the liquid. If gas wants its molecules to stick together due to static electricity buildup, then it won’t want to break up into tiny droplets or mist and mix readily and properly with the oxygen in our air/fuel mixture. Poor mixture equals poor energy—simple as that. Rapid and thorough atomization into a more efficient combustible mixture is essentially the value of removing static cling from fuel. The benefits to your engine are not of the same ilk as adding a cam or bumping compression, to increase power. What happens instead is you get a whole lot closer to the power potential that cam change or higher compression has to offer. Stock engines, built engines, it don’t matter—you’ll feel the difference of more energy emanating from the improvement in conflagration.
“More throttle in the bottle”
That’s the slogan on every bottle of Oxytane. It’s no empty boast either. Fifteen years in the making, this stuff does for fuel what dryer sheets do for socks—namely unstick the static! The formula is as mysterious as that of Coca-Cola or Colonel Sander’s 11 herbs and spices, but that’s to be expected. After all, so far, it’s the only product in the world that can do what it does. What does it do? Well, thanks for asking! Besides the good stuff we’ve already touched on, such as improved energy output (more power), livelier throttle response, notably better fuel economy, and hugely improved emissions, there’s a reduction in deposits within the engine, and that means longer intervals between overhauls, as well as an easier life for the engine components, since they can do more work with less effort. Particularly beneficial to air-cooled H-D engines, that also means cooler operating temperatures. In other words, if you feel like you can’t win with modern fuel, you can’t lose with Oxytane—so go with the flow! www.oxytane.com.